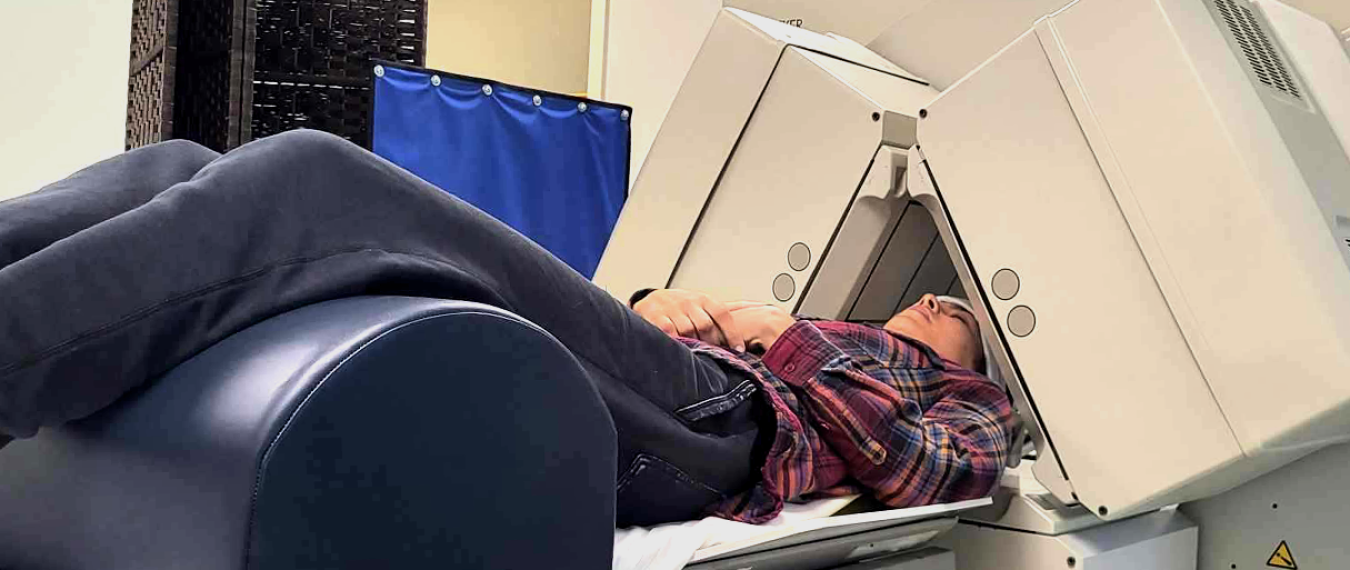
"My brain no longer worked"
On June 28th 2023, exactly at 3:00 PM, I walked into a psychiatric office full of trust and optimism. I wasn’t in a crisis. I wasn’t struggling to function. In fact, I was training for a marathon. I had my favorite running shorts on and a pair of bright shoes. I felt strong, hopeful, and grounded.
What brought me there was a recommendation from my therapist, who had noted a mild cyclical pattern in my mood. She suggested I consult a psychiatrist just to explore options, and that single meeting led to the psychiatrist telling me that unresolved trauma would make me a poor mother and that TMS was my last chance.
I hadn’t scored high on depression or anxiety screenings. I had no history of psychiatric medications. But the psychiatrist’s warning hit something tender in me, and I trusted what she said. I was told that my cyclical pattern of mood that the therapist noted was due to an “overactive amygdala.” I was told TMS – Transcranial Magnetic Stimulation – was cutting-edge, incredibly safe and could help. I read the brochures that promised an 83% success rate, no systemic side effects, and relief without medication. I remember thinking it was the smartest and most proactive choice I could make for my future.
I was wrong.
The moment the machine started on my first session, I felt sharp, jarring pain in my skull. Not pressure. Not discomfort. Pain. I burst into tears after the 19-minute session, but the staff reassured me. They told me it was normal and that I was probably just overly sensitive. They said it would pass. They said to keep going.
By the second session, I could barely get out of bed. I was dizzy and nauseous, and my balance was off. By the third session, they gave me a silk cap and adjusted the settings. They told me I needed to adapt to the treatment. But my brain and body were clearly screaming “no”. And yet, I still almost listened to them instead of myself. Almost.
After I stopped, I began losing functions that had felt effortless days before. I couldn’t drive. I couldn’t read. I couldn’t follow conversations. My vision doubled. I dropped things constantly. I tripped walking up and down the stairs. It felt like my nervous system had been scrambled and my brain no longer worked the same way. I was in a fog, unable to name what had happened, but I knew something in me had been injured.
I did what any of us would do. I asked for help. I told doctors and specialists everything. The sharp pain, the dizziness, the vision issues, the fog. I submitted MRI, MRA, and CT scans. But because the imaging was mostly “clear” my symptoms were dismissed. One doctor called the abnormal blood vessels on the left side of my neck that were viewed via the MRA congenital.
The psychiatrist that did the harm told me maybe I had a pre-existing condition. At my primary care office, I was called “headache girl.” No one took the broader cognitive dysfunction seriously.

PT Assessment: I met with several PT’s before I found one that truly understood the trauma of a brain injury and the link between TMS and my symptoms. This image is from one of the first assessments where I was told that I had deficits, but the why and how to heal was gravely misunderstood.
I reached out to NeuroStar, the manufacturer, and asked for my psychiatrist’s office to file an adverse event report. When I finally saw what they submitted, I was stunned. It was inaccurate and downplayed. I sent back clarifications, hoping to correct the record. I received no confirmation that my corrections were submitted or reviewed. I followed up. I was eventually told that most people just have headaches and they go away with time. No neurologist was consulted. A peer review wasn’t deemed necessary. The psychiatrist’s office, in the end, told me they had done all they could.
Later, I submitted a complaint directly to the FDA’s MAUDE database. Months after receiving confirmation that NeuroStar received my complaint from the FDA, I still have not heard a single word in response, even after I submitted more clarification that was requested. No confirmation. No further follow-up. No investigation. Nothing. The silence is louder than any rejection.
This is medical neglect. And, the gaslighting didn’t end there. When I tried to share my story online, I was silenced or shamed. I was told I was fearmongering. I was told that maybe I just had anxiety. I was told that I was standing in the way of others’ healing. In one mental health subreddit, I was even told that informed consent was a doctor’s job, not something patients should be responsible for clarifying. But if doctors aren’t being honest, and the system ignores harm, then where does that leave people like me?
Some even claimed I was making up my story to build a brand. That was the moment I realized how deep this resistance ran. The TMS community, including some practitioners, simply doesn’t want to believe that real harm is happening.
And yet as I have shared my story, I’ve discovered that I am not the only one. There are other people who have experienced similar harm from this treatment. People who have lost jobs, relationships, mobility, and even their sense of identity. People whose stories are too easily dismissed as rare, defective, or imagined.
My experience with TMS has led me to take a deep dive into the research on how TMS was cleared by the FDA, the training of the techs administering it, patient screening, and its off-label uses. Drawing on the analysis from the National Center for Health Research⁽¹⁾, what I found is troubling: I realized the system that was supposed to protect me had serious gaps. TMS never went through the rigorous Class III approval process required for high-risk brain devices. Instead, it was cleared through a regulatory loophole using both the de novo process and the 510(k) pathway⁽²⁾, claiming it was "substantially equivalent” to devices that don’t even stimulate the brain cortex. Attempts to compare it to Electrocompulsive Therapy (ECT), a class III device, were rejected because TMS did not meet that threshold⁽³⁾. Knowing this made my own experience feel even more precarious. I had been put in the hands of a treatment that had not been fully tested and was not as “safe” as they led me to trust.
Early TMS trials showed minimal benefit over sham treatment, yet post-hoc analysis excluded patients with multiple antidepressant failures and those who dropped out, making results appear more favorable. Based on this data, the FDA cleared TMS for patients who failed exactly one antidepressant which is an impractical guideline that does not follow real-world application.
I experienced firsthand the consequences of this flawed process and the confusion, pain and dismissal that followed. The FDA’s clearance of TMS created a false sense of safety, allowed harm to be hidden, and left those injured without validation. Despite being applied directly to the brain, TMS escaped scrutiny, putting patients like me at risk and making it harder for their injuries to be believed.
For me, the consequence of this was far too real and became painfully personal. The system that was supposed to help protect and heal was one that could not be relied on. That’s when I realized: healing wouldn’t come from medicine or the systems I thought would protect me, it would come from building my own path with a team of care providers who understood trauma, the brain, and the nervous system.

Brain Injury Landscape: A collection of images documenting my symptoms like dizziness, vision issues, pain, and fatigue and showcasing the daily reality of living with brain injury.
Healing after my TMS injury didn’t happen all at once or with just one treatment. It’s been a slow and sometimes frustrating process of finding people who really understand brain injury and trauma.
Here are some of the treatments that made a huge impact for me:
- Physical therapy focused on my upper neck helped ease pressure on my brainstem and improve my balance and neck strength.
- Getting my jaw aligned reduced muscle tension that was causing pain in my head.
- Vision therapy was a game changer because when your brain can’t make sense of what your eyes see, everything from walking to reading becomes exhausting and confusing.
- Speech therapy helped me find my words again and sharpen my focus during conversations.
- Integrative therapy taught me breathing exercises and visualization techniques that calmed my overwhelmed nervous system and eased my anxiety.
- Craniosacral therapy and UMAC acupuncture helped release deep tension in my body and emotions, giving me a sense of balance and safety that I hadn’t felt in a long time.
- Wearing trauma and prism glasses made light less painful and helped my eyes work together better.
- Syntonic light therapy gently supported my brain when I felt foggy and tired.
But healing was more than therapies. It was about learning to listen to my body, respect my limits, and find my voice after years of being dismissed. Nature, slow mornings, close friends, and even using AI tools to write when my brain was too foggy made a big difference.
Some days are still hard, and some symptoms linger. But I don’t blame myself anymore or stay silent. I trust what my body is telling me. I say no when I need to. I take up space in the world with more confidence than I thought possible. Healing has taught me patience, gentleness, and the power of truly listening which are lessons I now carry with me as I share my story.
If you’ve been harmed by TMS or any treatment, please know you’re not alone. If your pain is minimized, your story erased, or your symptoms ignored, please keep speaking. Your voice matters. Your story matters. We all deserve healing rooted in respect, truth, and care.
This is how I found my inner compass when every external one failed me.
Citations:
- National Center for Health Research. (n.d.). Is TMS proven effective for depression? Retrieved August 26, 2025, from https://www.center4research.org/is-tms-proven-effective-depression
- U.S. Food and Drug Administration. (2006). 510(k) Premarket notification: NeuroStar® TMS system (K061053) [PDF]. https://www.accessdata.fda.gov/cdrh_docs/pdf6/k061053.pdf
- U.S. Food and Drug Administration. (2008). 510(k) Premarket notification: NeuroStar® TMS system (K083538) [PDF]. https://www.accessdata.fda.gov/cdrh_docs/pdf8/K083538.pdf
- National Center for Health Research. (2011, January 26). Statement of the National Research Center for Women & Families at FDA Advisory Panel meeting regarding NeuroStar TMS system for major depression. https://www.center4research.org/statement-national-research-center-women-families-fda-advisory-panel-meeting-regarding-neurostar-tms-system-major-depression/